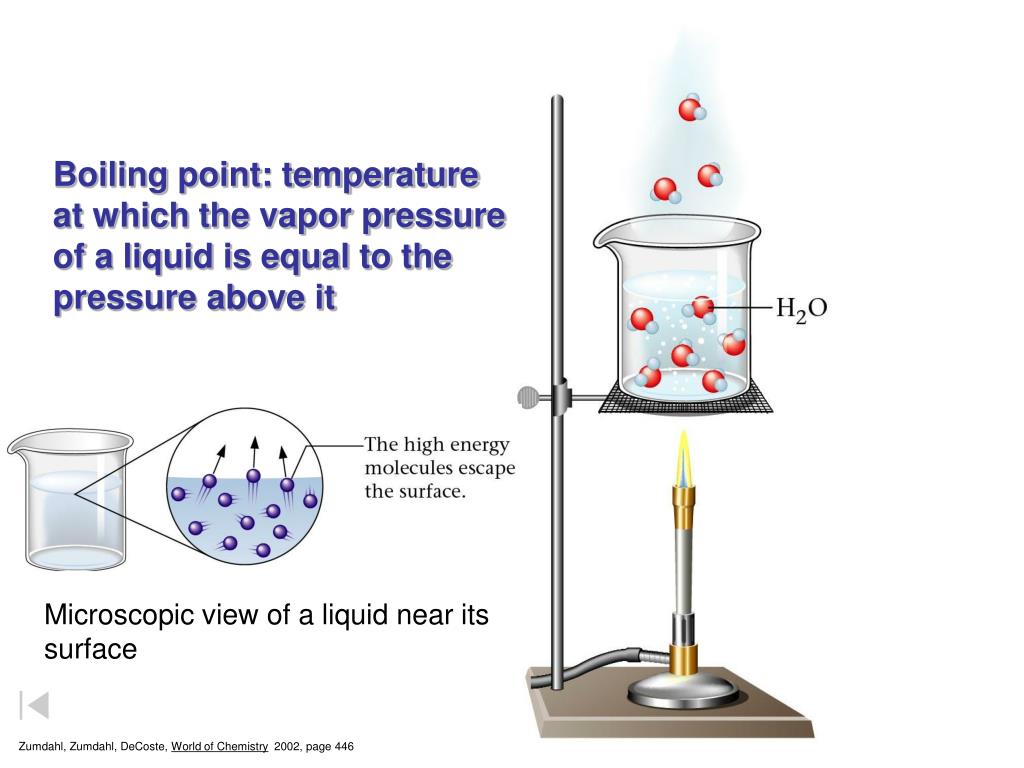
Why Does Water Vapor Pressure Increase With Temperature . How does the vapor pressure change with temperature? To understand that the relationship between pressure,. As temperature increases the molecular activity at the surface of the water would increase. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. We would expect it to get larger as the temperature. The effect of temperature on the saturated vapor pressure of water. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2:
from www.slideserve.com
As temperature increases the molecular activity at the surface of the water would increase. To understand that the relationship between pressure,. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The effect of temperature on the saturated vapor pressure of water. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. We would expect it to get larger as the temperature. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: How does the vapor pressure change with temperature?
PPT Intermolecular Forces PowerPoint Presentation, free download ID
Why Does Water Vapor Pressure Increase With Temperature We would expect it to get larger as the temperature. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. How does the vapor pressure change with temperature? We would expect it to get larger as the temperature. As temperature increases the molecular activity at the surface of the water would increase. To understand that the relationship between pressure,. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. The effect of temperature on the saturated vapor pressure of water.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation, free download ID352441 Why Does Water Vapor Pressure Increase With Temperature The effect of temperature on the saturated vapor pressure of water. As temperature increases the molecular activity at the surface of the water would increase. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: How does the vapor pressure change with temperature? To understand that the equilibrium vapor pressure of a liquid depends. Why Does Water Vapor Pressure Increase With Temperature.
From www.caee.utexas.edu
Vapor Pressure vs. Temperature Why Does Water Vapor Pressure Increase With Temperature To understand that the relationship between pressure,. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: As. Why Does Water Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Why Does Water Vapor Pressure Increase With Temperature To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: As temperature increases the molecular activity at the surface of the water would increase. To understand that the relationship between pressure,. We would expect it. Why Does Water Vapor Pressure Increase With Temperature.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry Why Does Water Vapor Pressure Increase With Temperature To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. We would expect it to get larger as the temperature. How does the vapor pressure change with temperature? To understand that the relationship between pressure,. As temperature increases the molecular activity at the surface of the water would increase. Vapor pressures. Why Does Water Vapor Pressure Increase With Temperature.
From www.myxxgirl.com
Water Vapor Pressure Chart Psi My XXX Hot Girl Why Does Water Vapor Pressure Increase With Temperature Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: As temperature increases the molecular activity at the surface of the water would increase. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. The effect of temperature on the saturated vapor pressure of water. To understand. Why Does Water Vapor Pressure Increase With Temperature.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Why Does Water Vapor Pressure Increase With Temperature Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: How does the vapor pressure change with temperature? The effect of temperature on the saturated vapor pressure of water. We would expect it to get larger as the temperature. As temperature increases the molecular activity at the surface of the water would increase. To. Why Does Water Vapor Pressure Increase With Temperature.
From partmcveighinjudicial.z21.web.core.windows.net
Phase Diagram Generator Why Does Water Vapor Pressure Increase With Temperature We would expect it to get larger as the temperature. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: As temperature increases the molecular activity at the surface of the water would increase. The. Why Does Water Vapor Pressure Increase With Temperature.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Why Does Water Vapor Pressure Increase With Temperature How does the vapor pressure change with temperature? Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To understand that the relationship between pressure,. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. As temperature increases the molecular activity at the surface of the water. Why Does Water Vapor Pressure Increase With Temperature.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Why Does Water Vapor Pressure Increase With Temperature The effect of temperature on the saturated vapor pressure of water. To understand that the relationship between pressure,. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To understand that the equilibrium vapor pressure of a. Why Does Water Vapor Pressure Increase With Temperature.
From www.numerade.com
SOLVED Over a water surface, the saturation vapor pressure (es) is Why Does Water Vapor Pressure Increase With Temperature To understand that the relationship between pressure,. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. We would expect it to get larger as the temperature. The effect of temperature on the saturated vapor pressure of water. As temperature increases the molecular activity at the surface of the water would increase. To. Why Does Water Vapor Pressure Increase With Temperature.
From www.chemistrylearner.com
Saturated Vapor Pressure Definition and Temperature Effect Why Does Water Vapor Pressure Increase With Temperature We would expect it to get larger as the temperature. The effect of temperature on the saturated vapor pressure of water. To understand that the relationship between pressure,. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure. Why Does Water Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Partial Pressure PowerPoint Presentation, free download ID6502396 Why Does Water Vapor Pressure Increase With Temperature The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. As temperature increases the molecular activity at the surface of the water would increase. To understand that the relationship between pressure,. The effect of temperature on the saturated vapor pressure of water. Vapor pressures have an exponential relationship with temperature and always increase. Why Does Water Vapor Pressure Increase With Temperature.
From www.numerade.com
From the plot of vapor pressures vs temperature above, estimate the Why Does Water Vapor Pressure Increase With Temperature How does the vapor pressure change with temperature? To understand that the relationship between pressure,. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Vapor pressures have an exponential relationship with temperature and. Why Does Water Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Does Water Vapor Pressure Increase With Temperature To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. As temperature increases the molecular activity at the surface of the water would increase. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: The effect of temperature on the saturated vapor pressure of water.. Why Does Water Vapor Pressure Increase With Temperature.
From imathworks.com
[Physics] Why is there any vapor above the water surface at atmospheric Why Does Water Vapor Pressure Increase With Temperature The effect of temperature on the saturated vapor pressure of water. We would expect it to get larger as the temperature. How does the vapor pressure change with temperature? To understand that the relationship between pressure,. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. To understand that the equilibrium vapor pressure. Why Does Water Vapor Pressure Increase With Temperature.
From www.sohu.com
【物理科普】歌词里的【105度的水】,可以用物理解释吗?_沸点 Why Does Water Vapor Pressure Increase With Temperature How does the vapor pressure change with temperature? The effect of temperature on the saturated vapor pressure of water. As temperature increases the molecular activity at the surface of the water would increase. To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. To understand that the relationship between pressure,. We. Why Does Water Vapor Pressure Increase With Temperature.
From respuestas.me
¿Por qué hay vapor sobre la superficie del agua a presión atmosférica y Why Does Water Vapor Pressure Increase With Temperature Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: The effect of temperature on the saturated vapor pressure of water. As temperature increases the molecular activity at the surface of the water would increase. The graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. How does. Why Does Water Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID1025977 Why Does Water Vapor Pressure Increase With Temperature How does the vapor pressure change with temperature? To understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Vapor pressures have an exponential relationship with temperature and always increase as temperature increases (figure 2: To understand that the relationship between pressure,. The graph shows how the saturated vapor pressure (svp) of. Why Does Water Vapor Pressure Increase With Temperature.